In the industry most of the time, it is important for the NDT inspector to be able to locate the source of the problem when it exists, or even when a fault is not located, know that the possibility of one exists. An understanding of the materials, the processes, and the possible interactions between them is, therefore, a must for an NDT Inspector. Hence in this article, we will learn about Engineering material and a brief about different types of Engineering Materials.
To learn about Career in NDT read our Blog:
Beginners Guide to Non-Destructive Testing (NDT) – Updated 2020
What is Material?
Material is a substance or mixture of substances that constitute an object.
Materials can be pure or impure, living, or non-living matter. They can be classified based on their physical and chemical properties, or on their geological origin or biological function. Materials science is the study of materials and their applications.
What are the engineering materials?
Engineering materials refers to the group of materials that are used in the construction of man-made structures and components. The primary function of an engineering material is to withstand applied loading without breaking and without exhibiting excessive deflection.
The major classifications of engineering materials include metals, polymers, ceramics, and composites which are further divided into many types.
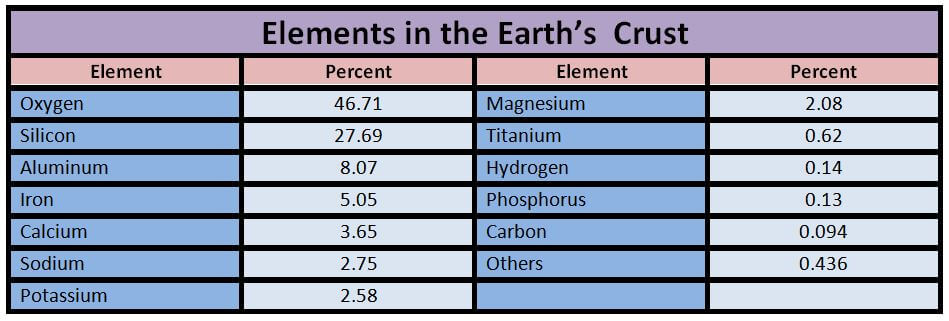
Engineering Materials which we will discuss in this article are :
Metals:
- Ferrous
- Non-ferrous
Non-Metals:
- Ceramics
- Glasses
- Plastics/polymers
- Elastomers
Metals
A metal is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well.
Metals are typically malleable (they can be hammered into thin sheets) or ductile (can be drawn into wires). A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride.
A metallic solid is one that has free electrons available in the structure to carry a current and that has a negative coefficient of conductivity with increasing temperature.
Metals can be classified as
- Ferrous
- Non-Ferrous
Ferrous Metals
“ferrous” means generally “containing iron“. The word is derived from the Latin word Ferrum (“iron”). Ferrous metals include steel and pig iron (with a carbon content of a few percent) and alloys of iron with others.
Ferrous materials containing iron, and steel is the most important material of this family. Because of availability, low cost, high strength, ease of fabrication into many shapes, and a wide range of properties, steel accounts for 80% of all metallic materials. It is widely used in manufacturing, building and construction industries.

Some common ferrous metals include
Alloy steel – steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight.
Carbon steel, also known as structural steel. Carbon steel is a steel with carbon content up to 2.1% by weight.
Cast iron – a group of iron-carbon alloys with a carbon content greater than 2%.
wrought iron– an iron alloy with a very low carbon (less than 0.08%) content in contrast to cast iron (2.1% to 4%).
Ferrous metals have high carbon content which generally makes them vulnerable to rust when exposed to moisture. There are two exceptions to this rule: wrought iron resists rust due to its purity and stainless steel is protected from rust by the presence of chromium.Most ferrous metals are magnetic which makes them very useful for motor and electrical applications.
Ferrous metals are used in shipping containers, industrial piping, automobiles, railroad tracks, and many commercial and domestic tools.
Nonferrous Metals
Non-ferrous metal and alloys, does not contain iron (ferrite) in appreciable amounts. Non-ferrous metals were the first metals used by humans for metallurgy. Copper was the first metal to be forged.
Important non-ferrous metals include
- Aluminum, copper, lead, nickel, tin, titanium, and zinc,
- Precious metals such as gold, silver and platinum and exotic or
- Exotic or rare metals such as cobalt, mercury, tungsten, beryllium, bismuth, cerium, cadmium, niobium, indium, gallium, germanium, lithium, selenium, tantalum, tellurium, vanadium, and zirconium.

Photo by Oleksandr Kurchev on Unsplash
General Applications of Nonferrous metals
Aluminum is used in the manufacturing of equipment for chemical and food industries, cooking utensils, cookers, steam-jacketed kettles, etc. Due to its lightweight and high tensile strength, it is used in the structural work of airplanes, ships, trains, buses, trucks, etc. And also used for roofing, sheathing, window frames, foils, posts, etc.
Copper is used in making cables and wires for electric applications. electroplating. manufacturing of utensils and making of copper alloys. making of munitions and tubes in engineering applications.
Zinc ,it has applications in the automobile, construction and shipbuilding industries, light industry, machinery, household electrical appliances, batteries and other industries.
Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. magnesium ignites easily in air and burns with a bright light, it’s used in flares, fireworks and sparklers.
Non-Metals
In chemistry, a nonmetal is a chemical element that mostly lacks the characteristics of a metal. Physically, a nonmetal tends to have a relatively low melting point, boiling point, and density. A nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity
Some Types of Non-metals
- Ceramics
- Glasses
- Plastic
- Elastomers
Ceramics
A ceramic is a solid material comprising an inorganic compound of metal or metalloid and non-metal with ionic or covalent bonds.
Ceramic engineering is the science and technology of creating objects from inorganic, non-metallic materials. This is done either by the action of heat or at lower temperatures using precipitation reactions from high-purity chemical solutions.
Properties of Ceramics:
Compared to metals and plastics, ceramics are hard, non-combustible, and inert. Ceramic materials are usually ionic or covalent bonded materials and can be crystalline or amorphous,have high melting points,Good hardness, strength and durability, have Low electrical and thermal conductivity.

Some Applications of Ceramics
Ceramics can be used in high temperature, corrosive and tribological applications. Engineering ceramics, such as silicon nitride, silicon carbide, and a large number of oxides, are used in industries ranging from aerospace to automotive and biomedical to electronics.
- Diamond (the synthetic variety)- is the ultimate engineering ceramic; it has for many years been used for cutting tools, dies, rock drills, and as an abrasive.
- Tiles – Our roofs, bathrooms, and kitchens are covered in ceramic tiles.
- Cookware – Majority of crockery and pots are made from ceramic
- Brick – Our homes are made from brick and are held together by cement, both of which are types of ceramic.
- Sanitary ware – toilets, sinks, and bathtubs are made from ceramic.
- Space NASA’s Space Shuttle had thousands of heat-resistant tiles protecting its exterior from overheating due to friction generated by Earth’s atmosphere.
- Artificial Bones and Teeth
Toughened ceramic is used in hip replacements and is designed to be porous so that it can stimulate natural bone growth around the artificial joint.
Glasses
Glass is a non-crystalline, amorphous solid. Glass is most often formed by rapid cooling (quenching) of the molten form; some glasses such as volcanic glass are naturally occurring.
Usually, glass is brittle and will fracture, unless it is laminated or tempered to enhance durability, typically inert, resistant to chemical attack, and can mostly withstand the action of water, have low ductility; they are very brittle, have low thermal conductivity compared to metals, can be good electrical insulators.
key Optical properties of glasses refractive index, dispersion, and transmission, making them superior to clear plastics for lenses, sight glasses and windows.
Types of glass
- Silicate glass.
- Soda-lime
- glass Borosilicate glass
- Lead glass
- Aluminosilicate glass
- Glass-ceramics
- Fibreglass
- Silica-free glass
General Applications of glass :
- lenses, windows, mirrors, and prisms.
- manufacturing optical lenses, prisms, and optoelectronics materials.
- Extruded glass fibers have applications as optical fibers in communications networks, thermal insulating material when matted as glass wool so as to trap air, or in glass-fiber reinforced plastic (fiberglass).
- Packaging applications like jars for food, bottles for drinks, flacon for cosmetics, and pharmaceuticals, drinking glasses, plates, cups, bowls.
- windows, facades, conservatory, insulation, reinforcement structures in Housing, and buildings.
- Interior design and furniture -mirrors, partitions, balustrades, tables, shelves, lighting.
- Appliances and Electronics -oven doors, cooktop, TV, computer screens, smart-phones.
- Automotive and transport windscreens, backlights, lightweight reinforced structural components of cars, aircraft, ships, etc.
- Medical technology, biotechnology, life science engineering, optical glass
- Radiation protection from X-Rays (radiology) and gamma-rays (nuclear)
- Renewable energy (solar-energy glass, wind turbines)
Plastics/Polymers
Chemically plastics are called polymers.
IUPAC (International Union of Pure and Applied Chemistry) definition of plastic.
A polymer is a substance composed of macromolecules. A macromolecule is a molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetitions of units derived, actually or conceptually, from molecules of low relative molecular mass.
The smallest unit structure or molecule that identifies the chemical involved is called a monomer. A polymer is a large molecule, or macromolecule, composed of many repeated monomers.
Polymerization is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks.

Photo by Ryan Quintal on Unsplash
Types of plastics
Long Chain polymers- thermoplastic polymers is a plastic polymer material that becomes moldable at a certain elevated temperature and solidifies upon cooling.
Commonly used thermoplastic plastics
- ABS – pipe, appliance cabinets, football helmets, handles, gears, impellers, plumbing hardware.
- Polycarbonate: gears, hydraulic fittings, coil forms, appliance parts, electronic components.
- Polyethylene: housewares, pipe, pipe fittings, squeeze bottles, sports goods, electrical insulation.
- Polystyrene: Toys, electrical parts, battery cases, light, fixtures, rigid conduits.
- Nylon: gears, cams, bearings, pump parts, coil forms, slide fasteners, gaskets, high-pressure tubing.
Cross-linked Polymers- Thermosetting Polymers is a polymer that is irreversibly hardened by curing of a soft solid or viscous liquid prepolymer or resin.
Commonly used Thermosetting plastics
- Epoxy: used in chemical tanks, pipe, printed circuit bases, short-run dies, pressure vessels.
- Melamine: Dinnerware, electrical components, table, and contour tops
- Polyester: electrical hardware, poker chips, toys, buttons, appliance cabinets, thermal insulation, table and countertops, ablative structural shapes.
- Silicon: high-temperature electrical insulation, high-temperature laminates, gaskets, bushings, seals, spacers.
- Urea: colored electrical parts, buttons, dinnerware.
Elastomers
Rubber-like solids with elastic properties are called elastomers. An elastomer is a polymer with viscoelasticity (i.e., both viscosity and elasticity) and has very weak intermolecular forces.
IUPAC defines the term “elastomer” by “Polymer that displays rubber-like elasticity.”
Polymer chains are held together in these materials by relatively weak intermolecular bonds, which permit the polymers to stretch in response to macroscopic stresses. Elastomers are usually thermosets (requiring vulcanization) but may also be thermoplastic.
Vulcanization Is curing of elastomers. It works by forming cross-links between sections of the polymer chain which results in increased rigidity and durability, as well as other changes in the mechanical and electrical properties of the materials.

Photo by Markus Spiske on Unsplash
Types of elastomers and their common applications.
- Natural rubber-used to produce automotive products, wearable items like footwear and rubberized fabrics, latex products, and anti-vibration materials.
- Polyurethanes: polyurethanes are used extensively in the textile industry to produce elastic clothing like spandex. However, thermoplastic polyurethanes are also used to manufacture shoes, cushions, cables, seals, and technical parts.
- Polybutadiene: production of automotive tires.
- Neoprene: production of corrosion-resistant gaskets, hoses, and coatings. Most famously, however, neoprene is used in wetsuits.
- Silicone rubber: commonly used in automotive, aerospace, medical, food production, and consumer product applications.
Conclusion
There are numerous numbers of engineering materials in the industry. we have to tried to cover the commonly used used basic types of engineering materials.
The purpose of NDT is to locate various faults in materials and products that have been processed to at least some degree. The flaws, defects or imperfection that may be located by inspection may be a result of the original material, caused by the processing used, created by some human error, or be a result of some combination of these.
NDT personnel need familiarity with many material characteristics in order to evaluate their suitability for service, through nondestructive testing. Determining the source or cause of defects is frequently necessary in order to eliminate these defects from production parts. Often the causes of defects lie in early farms or stages of material production or processing and in some cases these prior processes control the response of materials to later processes, during which defects or failures are induced.
References
- TWI global,
- Wikipedia.org
- Sciencedirect.com
- Materials and Processes for NDT Technology-ASNT
- https://www.glassallianceeurope.eu/
- Feature Photo by Robby McCullough on Unsplash

Hi! I could have sworn I’ve visited this web site before but after looking at many of the posts I realized it’s new to me. Nonetheless, I’m definitely delighted I stumbled upon it and I’ll be book-marking it and checking back frequently!
Very informative article.Really thank you! Great.
I value the article post.Really looking forward to read more. Much obliged.
Muchos Gracias for your article post.Thanks Again. Much obliged.
I really liked your blog article.Much thanks again. Fantastic.
Major thankies for the post.Much thanks again. Great.
I really like and appreciate your post.Much thanks again. Want more.
Thanks so much for the article.Much thanks again. Cool.
Pingback: Introduction to Casting Process | World Of NDT
Pingback: Metal Joining Technology | World Of NDT
Pingback: World Of NDT Mechanical properties of metals | World Of NDT